Carbon Dioxide (CO2): the Good & the Bad
- Sylvia Rose

- Mar 8, 2025
- 4 min read
Updated: Mar 17, 2025
Carbon dioxide (CO2) is a colorless, odorless gas essential for life on Earth. CO2 is both a natural component of the atmosphere and a byproduct of human activity. Destruction erupts when levels reach a tipping point.

A simple molecule, carbon dioxide is composed of one carbon atom and two oxygen atoms. It exists naturally in the atmosphere, making up a small 0.04% of air.
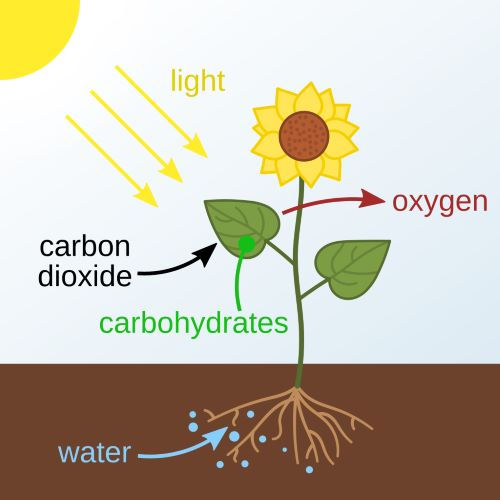
Created through various natural processes such as respiration, combustion and volcanic eruptions, it's needed by plants for photosynthesis. Plants consume CO2 to produce sugars for energy, with oxygen as waste.

Carbon dioxide levels are quantified in parts per million (ppm). Before the Industrial Revolution, atmospheric CO2 levels are around 280 parts per million (ppm). Current atmospheric levels are over 415 ppm.
This level is unprecedented in at least 800,000 years. Ice core data shows CO2 levels fluctuate over Earth's history. The current surge is due largely to human activity.
Natural processes like weathering and environmental absorption help control CO2. The current rate of emissions overwhelms these systems.

Carbon Dioxide (CO2): The Good
Photosynthesis
CO2 is the lifeblood of plants. Through photosynthesis, plants absorb CO2 from the atmosphere and, with the help of sunlight and water, convert it into glucose, fructose and oxygen.
This process is the basis of the food chain and provides the oxygen breathed by organisms such as humans. Without CO2, plants would cease to exist, and consequently, animals.
When organisms inhale oxygen, they exhale CO2, which is recycled into the atmosphere. Plants take it up and the cycle continues.
A single mature tree absorbs 4.5 - 22 kg (10 - 48 lb) of CO2 per year, depending on species and maturity of tree. This contributes greatly to atmospheric CO2 reduction.
Natural carbon sinks such as oceans and forests absorb more CO2 than they release. Forests, especially tropical rainforests, are especially good at sequestering carbon.
Deforestation releases the stored carbon into the atmosphere. Oceans absorb about 25% of the CO2 emitted by human activities each year. Excess carbon emissions overburden natural sinks like forests and oceans.

Regulating Earth's Temperature
CO2 acts as a blanket, trapping some of the sun's heat in the atmosphere. This natural greenhouse effect keeps the Earth warm enough to support life. Without it, the Earth would be a frozen wasteland. Too much is too bad.
Industry
Carbonating beverages, preserving food and production of some food additives uses CO2. When CO2 is dissolved in water under pressure, it creates the fizz of drinks like soda and sparkling water.

CO2 is extensively used in food preservation methods like modified atmosphere packaging. Packaging fresh produce in a CO2-rich environment can extend its shelf life.
CO2 is used in fire extinguishers and for producing chemicals like urea and methanol. It's also used in making dry ice and as a coolant in refrigeration.

Carbon Dioxide (CO2): The Bad
The rapid increase in atmospheric CO2 levels is considered to exacerbate climate change. Burning of fossil fuels such as coal, oil, and natural gas is the primary source of excess CO2 emissions.
These fuels release carbon which has been stored underground for millions of years. CO2 is injected into oil wells to increase oil production.
Ocean Acidification
As the ocean absorbs excess CO2, it becomes more acidic and destroys marine life including shellfish and coral reefs, which rely on calcium carbonate.

Extreme wildfires: According to NASA scientists, extreme forest fires in 2023 release about 640 million metric tons of carbon.
Disruption of Ecosystems
Altered weather patterns and rising temperatures disrupting ecosystems.
Rising atmospheric CO2 alters species distributions and interactions. Some species may thrive in warmer conditions, while others struggle to adapt.
Future Attempts at Carbon Sequestration & Capture
Technologies are being developed to capture CO2 emissions from industrial sources and store them underground, as in carbon sequestration. They may also be used in other industrial processes, a technique known as carbon capture and use.

READ: Lora Ley Adventures - Germanic Mythology Fiction Series
READ: Reiker For Hire - Victorian Detective Murder Mysteries


