Tartrate Crystals: Secrets of Tartaric Acid
- Sylvia Rose

- Oct 2, 2024
- 3 min read
Tartrate crystals appear on the bottom of a wine glass or in a bottle of grape juice, created by a reaction of tartaric acid. Reference to these creations appears in alchemical and other recipes as red or white tartar, the second being an ingredient in baking powder.

Tartrate Crystals and Tartaric Acid
Tartrate crystals form in wine and grape juice due to the presence of tartaric acid. Tartaric acid is a naturally occurring organic acid found in grapes, cherries, avocado, citrus and more. It's influential in determining the acidity of wine.
When wines or grape juices are chilled, the tartaric acid combines with potassium to form tartrate crystals. These crystals are known as wine diamonds. They're safe to consume and purveyed medicinally or used in processes by ancient alchemists and physicians.

What Are Tartrate Crystals?
Tartrate crystals, also referred to as potassium bitartrate, are solid formations that occurring naturally in some wines and grape juices. They are a byproduct of tartaric acid, a key acid in grapes and some other fruits, with a crucial role in taste, stability, and preservation of wine.
What Is Tartaric Acid?
Tartaric acid is a naturally occurring organic acid most commonly found in grape juice. It constitutes most of the acidity in wine, influencing its flavor, stability and aging potential. Tartaric acid is essential for the formation of tartrate crystals, which appear when certain conditions such as temperature change cause crystallization of the acid.
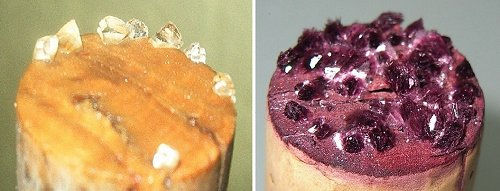
How Do Tartrate Crystals Form in Wine or Grape Juice?
Formation of tartrate crystals in wine or grape juice is a natural phenomenon. As wine or grape juice is cooled, solubility of tartaric acid decreases, leading to crystallization of tartrate crystals. These crystals typically cling to the sides or settle at the bottom of the container. The formation of tartrate crystals is intricately linked to the winemaking process.
How it happens:
Fermentation: During the fermentation process, yeast consumes the sugars present in grape juice and converts them into alcohol. One of the byproducts of this process is tartaric acid, which dissolves in the liquid.
Cooling and Aging: As the wine cools or ages, particularly in cold storage conditions, the solubility of tartaric acid in the liquid decreases. This is especially true for white wines that are often subjected to chilling.
Crystallization: When the solubility limit is reached, tartaric acid molecules begin to bond with potassium ions, forming potassium bitartrate. These molecules then crystallize out of the solution, resulting in visible tartrate crystals.

Health Benefits of Tartrate Crystals
Tartrate crystals health benefits include:
Natural Antioxidants: Tartaric acid possesses antioxidant properties, to help combat oxidative stress in the body. Antioxidants are known for their role in protecting cells and promoting overall health.
Digestive Aid: Tartaric acid can stimulate gastric juices, aiding digestion. Moderate amounts of tartar-rich beverages can improve gastrointestinal health.
Source of Potassium: Potassium bitartrate, the primary component of tartrate crystals, contains potassium, a vital mineral for heart health and muscle function.

Uses of Tartrate Crystals
Tartrate crystals have a variety of applications:
Culinary Uses: Tartaric acid is often used in baking as cream of tartar, a stabilizing agent for whipped egg whites and in preventing sugar crystallization in candies. It can also enhance flavors and textures in certain recipes.
Winemaking Practices: Some winemakers intentionally encourage the formation of crystals during the stabilization process, a technique known as “cold stabilization.” This helps improve the clarity and stability of the wine, ensuring a better product for consumers.
Industrial Applications: Tartrate crystals find uses in various industries, including in cosmetics, formulations, pharmaceuticals natural food preservatives.

To achieve cold stabilization, the wine's temperature is reduced to approximately -4°C to 0°C following fermentation for a duration of 1-2 weeks, facilitating precipitation of crystals. When conductivity stabilizes, the wine is filtered out of the vessel, leaving tartrate crystals in place.
Non-Fiction Books:
Fiction Books:
READ: Lora Ley Adventures - Germanic Mythology Fiction Series
READ: Reiker For Hire - Victorian Detective Murder Mysteries


